Cobenfy: Redefining Schizophrenia Treatment After 70 Years


Cobenfy: A Paradigm Shift in Schizophrenia Treatment
Cobenfy, also known as KarXT, is an oral antipsychotic for adults that was recently approved by the FDA. The drug combines xanomeline and trospium chloride, offering an innovative treatment that targets M1/M4 acetylcholine receptors, unlike traditional antipsychotics, which target dopamine receptors.
Cobenfy introduces a new mechanism of action for treating schizophrenia and is particularly notable for its potential to mitigate some of the side effects commonly associated with existing drugs. While dopamine receptor-targeting medications have been effective in relieving acute psychotic symptoms, their side effects—such as weight gain, sedation, and movement disorders—often make them unsuitable for all patients. Cobenfy provides a promising alternative, offering hope to many individuals.
Co-developed by Karuna Therapeutics and its parent company Bristol Myers Squibb (BMS), Cobenfy represents the culmination of their research collaboration. Notably, KarXT is the first FDA-approved drug to target cholinergic receptors, signifying a paradigm shift in the treatment of schizophrenia.
Novel mechanism of action
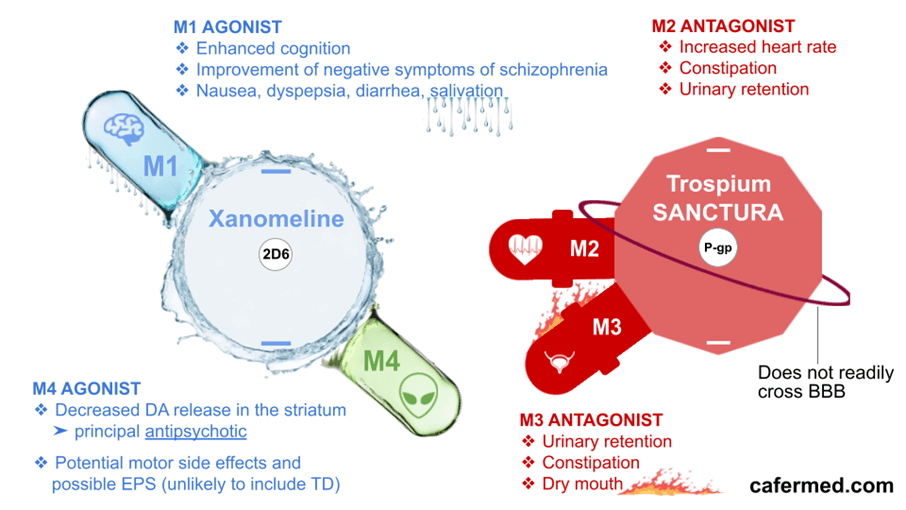
Cobenfy works by modulating neurotransmission through cholinergic pathways in the brain, effectively improving schizophrenia symptoms while reducing side effects. Xanomeline acts on M1 and M4 muscarinic receptors in the brain to reduce psychotic symptoms. Meanwhile, trospium does not cross the blood-brain barrier, minimizing gastrointestinal side effects by blocking muscarinic receptors in the peripheral nervous system.
Muscarinic receptors (M1/M4) are closely associated with schizophrenia pathology and are predominantly expressed in brain regions affected by the disorder. Xanomeline, an M1/M4-preferential agonist with excellent central nervous system penetration, has been shown in multiple studies to provide antipsychotic effects.
Thanks to this innovative approach, Cobenfy is emerging as an important alternative for patients who do not respond to existing treatments or who find it difficult to continue treatment due to side effects. It has been reported to be effective not only for positive symptoms, such as hallucinations and delusions, but also for negative symptoms, such as anhedonia and social isolation, expanding its potential applications in schizophrenia treatment.
Reliability of clinical study results
In two 5-week clinical studies, participants receiving COVENPI experienced a significant reduction in Positive and Negative Syndrome Scale (PANSS) scores compared to the placebo group. This demonstrates the drug's rapid onset of action, even within a short treatment period.
Results from the EMERGENT-2 trial (see https://pubmed.ncbi.nlm.nih.gov/38104575):
- 5-week double-blind, placebo-controlled trial in 252 patients
- Reduction in PANSS total score:
- Cobenpiv group: -21.2 points
- Placebo group: -11.6 points
- Statistically significant difference demonstrated (p<0.0001)
Results from the EMERGENT-3 trial (see https://pubmed.ncbi.nlm.nih.gov/38691387):
- 256 patients.
- Reduction in PANSS total score:
- Cobenpiv group: -20.6 points
- Placebo group: -12.2 points
- Effect size (Cohen's d): 0.60
Designed to reduce side effects
Cobenfy employs a dual mechanism of action, with its two primary components fulfilling distinct roles. Xanomeline improves symptoms by activating M1/M4 muscarinic receptors in the central nervous system, while trospium blocks peripheral muscarinic receptors, reducing side effects such as digestive issues and constipation. This design has proven effective in minimizing serious side effects commonly seen with traditional antipsychotic medications.
In clinical trials, Cobenfy has generally been associated with only mild side effects, with few serious adverse effects observed. However, due to the risk of liver damage, Cobenfy is contraindicated in patients with moderate or severe hepatic dysfunction and in those with a history of encephalopathy.
Industry impact and future outlook
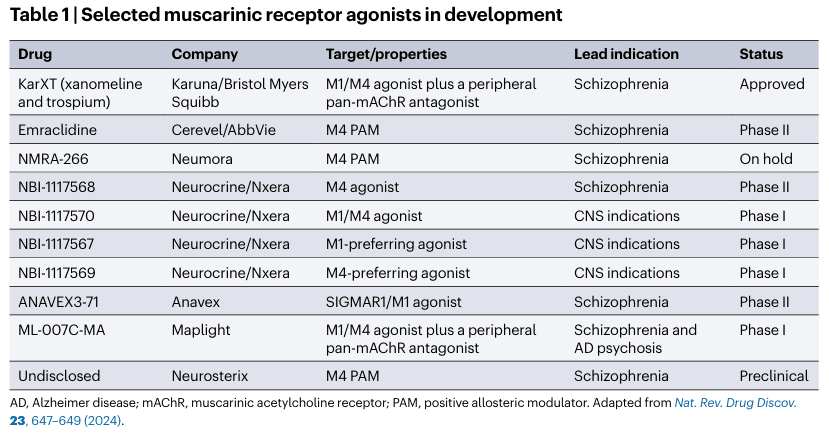
The approval of Cobenfy validates the muscarinic approach to schizophrenia treatment and has sparked significant investment in the pharmaceutical industry. In early 2024, BMS acquired Karuna Therapeutics for $14 billion, and AbbVie invested $8.7 billion to acquire Cerevel Therapeutics, which is developing a competing drug, emeraldine. Analysts project that Cobenfy could achieve peak annual sales of $6 billion to $10 billion.
Currently, researchers are exploring combinations of existing dopamine receptor blockers with muscarinic agonists to enhance treatment effectiveness, as 75–80% of schizophrenia patients struggle with adequate symptom management. A Phase 3 clinical trial investigating Cobenfy as an adjunctive therapy is underway, with top-line results expected in early 2025.
Cobenfy in a variety of conditions
BMS is also testing Cobenfy as a treatment for other conditions, such as psychosis associated with Alzheimer's disease. Additionally, a range of mAChR-targeting drugs, including M1-preferred agonists, M4-preferred agonists, and positive allosteric modulators, are currently in clinical development.