Novel drugs first shown at EFMC-ISMC 2024


New drugs first shown at EFMC-ISMC 2024
With so many medicinal chemists in attendance at EFMC-ISMC 2024, there was a lot of sharing of research achievements, but two First Disclosure sessions stole the show. These sessions revealed the structures of the latest clinical candidates for the first time. There are seven in total, and here's a quick look at what each one has to offer.
| Name | Target |
|---|---|
| HRX-215 | MKK4 |
| BAY 2413555 | M2 Receptor |
| NVP-EVS459 | Folate receptor |
| GSK484 | PAD4 |
| NVP-DFF332 | HIF-2α |
| CHF-6523 | PI3Kδ |
| AZD-4144 | NLRP3 Inflammasome |
1. Discovery and Development of a First in Class MKK-4 Inhibitor to Increase Liver Regeneration First Disclosure of the Clinical Candidate HRX-215
Prof. Stefan LAUFER (UNIVERSITY OF TÜBINGEN, Tübingen, Germany)
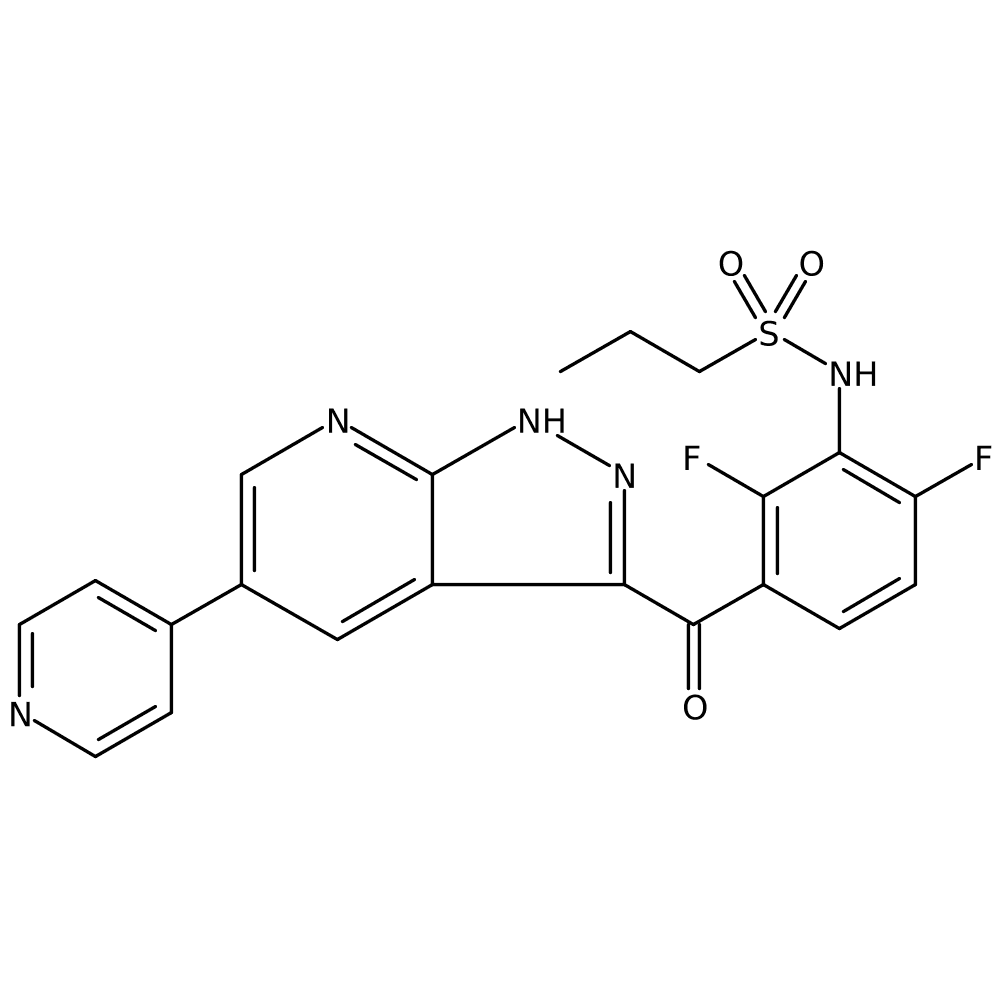
- This drug was developed to help patients at high risk of liver failure after liver resection surgery or liver transplant patients. Activates ATF2, ELK1 transcription factors that promote liver cell regeneration by inhibiting MKK4.
- Increased liver regeneration after liver resection in rat and pig models. Five of five pigs with 85% liver resection survived and exhibited normal behavior with HRX215 treatment, while five of six placebo group pigs were comatose and euthanized within 24 hours of surgery.
- The results of the Phase 1 clinical trial, completed in May 2022, showed that HRX215 is safe in humans and has excellent pharmacokinetic properties, and a Phase 2 is currently being planned.
*Source: https://www.cell.com/cell/fulltext/S0092-8674(24)00225-3
2. Discovery of BAY 2413555, First Highly Selective Positive Allosteric Modulator of the M2 Receptor to Restore Autonomic Balance in Heart Failure Patients
Dr Alexandros VAKALOPOULOS (BAYER, Wuppertal, Germany)
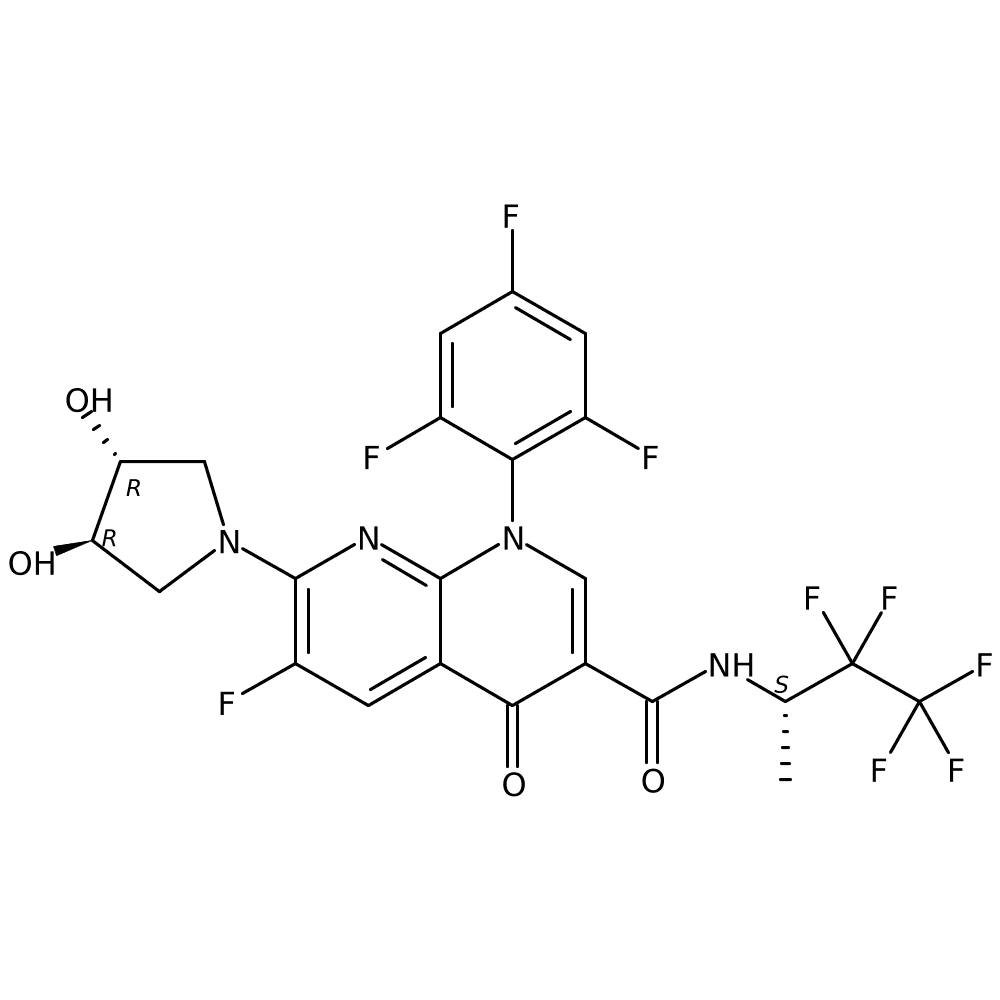
- It was developed to restore autonomic balance in patients with heart failure.
- It regulates heart rate through M2 receptors and restores autonomic balance by inhibiting sympathetic nerves and promoting parasympathetic nerves.
- Phase 1 ended in March 2023 and no further Phase 2 plans have been announced.
*source: https://clinicaltrials.bayer.com/study/20623/
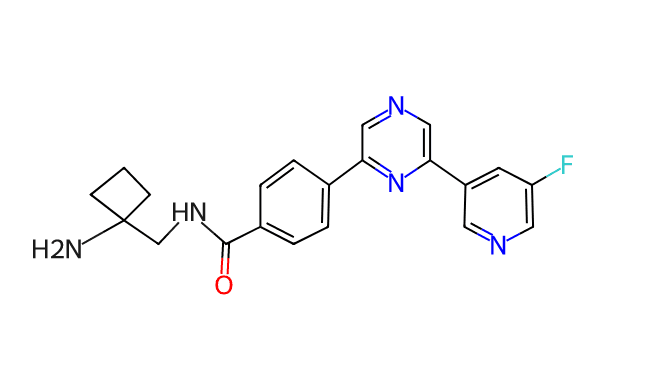
3. Discovery of NVP-EVS459, a Low Molecular Weight Folate Receptor Targeting Radioconjugate for the Treatment of Folate Receptor Positive Cancers with First-In-Class Potential
Dr Philipp HOLZER (NOVARTIS PHARMA AG, Basel, Switzerland)
- It is a promising drug for the treatment of cancers such as ovarian cancer and non-small cell lung cancer where the folate receptor is highly expressed.
- Cancer cells overexpress FR to absorb folate for rapid proliferation, and by combining the radioactive isotope lutetium-177 with EVS459, it delivers radiation directly to cancer cells to selectively destroy them.
- On September 6, 2024, the Phase 1 clinical trial began.
*source: https://www.careacross.com/clinical-trials/trial/NCT06376253
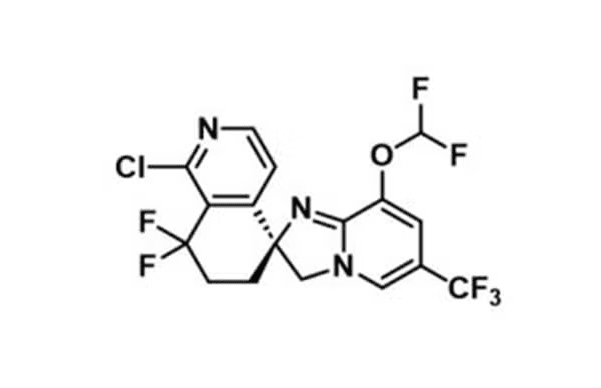
4. Discovery of Clinical Candidate GSK484: a Novel "Irresistible" Chemical Class for the Treatment of Malaria
Dr Jorge FERNANDEZ (GSK, Tres Cantos, Spain)
- Peptidylarginine deiminase 4 is a calcium-dependent enzyme, with an IC50 of 50 nM in the absence of calcium and 250 nM in the presence of 2 mM calcium.
- PAD4 is an important enzyme in the inflammatory response, catalyzing citrullination, the conversion of arginine to citrulline.
- This process plays an important role in the inflammatory response, regulation of the immune response, and formation of neutrophil extracellular traps, making PAD4 inhibition therapeutically beneficial by reducing the excessive inflammatory response during malaria infection.
*source: https://academic.oup.com/jleukbio/article/111/6/1235/6885198
5. Discovery of NVP-DFF332 as a Selective Inhibitor of the Transcription Factor Hypoxia-Inducible Factor-2ALPHA (HIF-2α)
Dr Robin FAIRHURST (NOVARTIS, Basel, Switzerland)
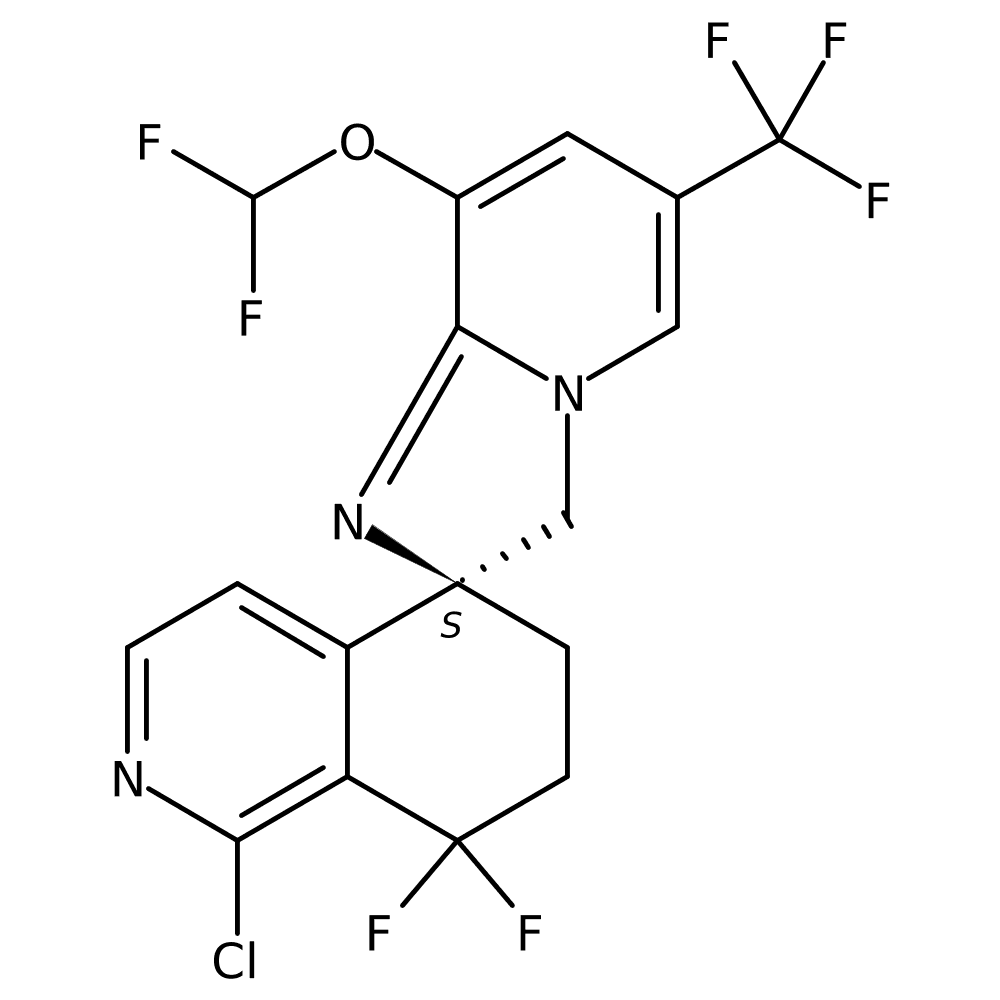
- It is an oral small molecule that inhibits HIF-2α and is being developed for clear cell renal cell carcinoma, ccRCC. This is an important protein that promotes the growth of cancer cells in a hypoxic environment and is highly expressed in ccRCC.
- In Phase 1, the disease control rate, or DCR, was 52.5%, with cancer not progressing or shrinking in size in more than half of patients.
- However, the trial is limited in that it was stopped due to a business decision before the optimal dose could be determined.
*source: https://ascopubs.org/doi/10.1200/JCO.2024.42.16_suppl.4513
6. First-time Disclosure of Clinical Candidate CHF-6523, an Inhaled Selective PI3Kδ Inhibitor for the Treatment of Chronic Obstructive Pulmonary Disease
Dr Paolo BRUNO (CHIESI FARMACEUTICI SPA, Parma, Italy)
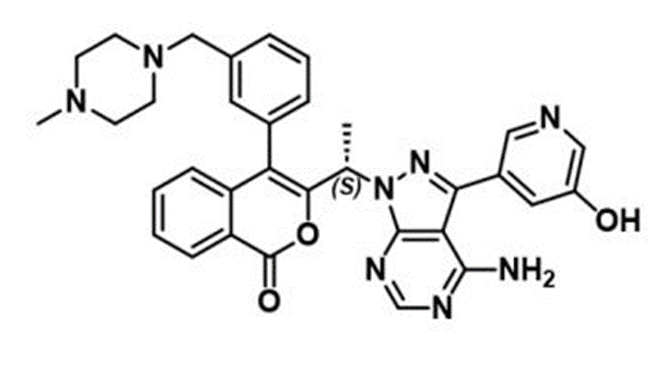
- It is available as a dry powder for inhalation.
- Inflammatory cytokines, such as IL-6, were measured in plasma and sputum to assess pre- and post-treatment changes.
- Phase 1 of the trial was completed in December 2022, and plans for Phase 2 have not yet been announced.
7. Inhibition of the NLRP3 Inflammasome is a Promising Concept to Treat a Variety of Inflammatory Diseases: AZD-4144
Dr Anders JOHANSSON (ASTRAZENECA, Molndal, Sweden)
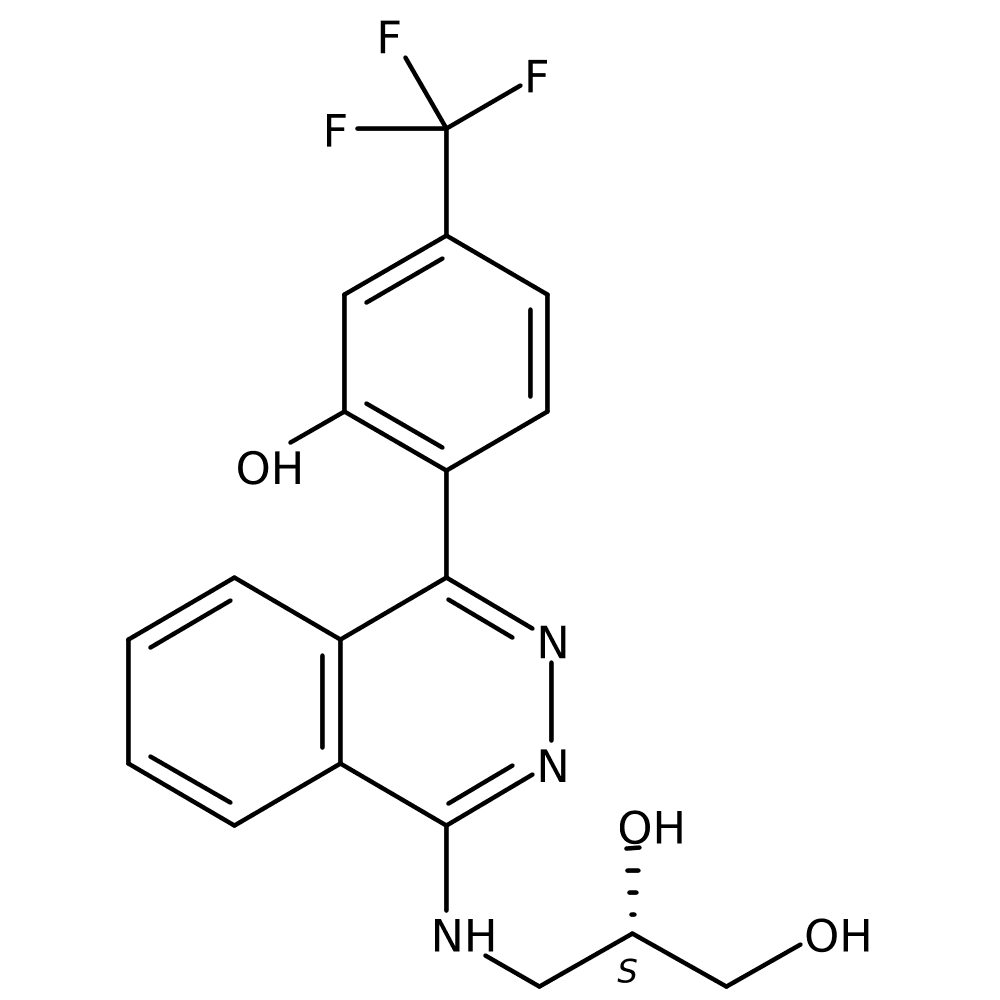
- The NLRP3 inflammasome is an important protein complex that activates pro-inflammatory cytokines such as IL-1β and IL-18 within cells, leading to an inflammatory response.
- Over-activation of this inflammasome is found in a variety of chronic inflammatory diseases, and AZD-4144 is currently being developed to treat inflammatory, cardiovascular diseases.
- It is currently in Phase 1 clinical trials, which are expected to be completed in 2025.
*source: https://www.astrazenecaclinicaltrials.com/study/D9440C00001/