AI로 발굴한 물질들의 개발 동향 살피기


AI로 발굴한 물질들의 개발 동향
AI가 신약 개발 경쟁으로 뛰어든 지 10년이 지났습니다. 그동안 AI 기반 신약 개발 플랫폼이 발굴한 신약 파이프라인은 어디까지 진행되었을까요? 이번 포스팅에선 신약 물질들의 표적은 무엇이었고, 그들의 개발 동향은 어떠한지에 대해 정리하고자 합니다.
Insilico Medicine이 쏘아 올린 작은 공
현재 알려진 AI 신약 개발 회사들은 약 500개 이상이며, 80% 이상이 딥러닝이 주요 트렌드가 된 2012년 이후 설립되었습니다. Atomwise, Exscientia, AbCellera, Flatiron Health, BenevolentAI, Recursion Pharmaceuticals 등이 대표적인 초기 AI 기업입니다. 이후 2014년 설립된 가장 주목할 만한 회사, Insilico Medicine이 등장합니다. Insilico Medicine은 2019년에 AI 기반 물질 발굴의 신호탄을 쏘아 올렸습니다. 단 21일 만에 DDR1을 표적으로 하는 분자를 탐색하고 in vitro 및 in vivo 예측을 성공적으로 끌어냈습니다. 같은 해 Deep Genomics 또한 AI 플랫폼으로 specific genetic mutation을 찾아냈고, 이를 표적으로 화합물을 디자인하여 18개월의 연구 끝에 DPG12P1를 발표했습니다.
2020-2022년에는 위에서 언급한 기업들의 후보 물질들이 전 임상 단계에 진입하면서, 2022년에는 수십 개의 AI로 발굴된 물질들이 임상시험에 진입하였습니다.
개발 동향
현재 2024년을 기준으로 대표적인 9개 기업의 타겟 및 진행 상황은 다음과 같습니다.
| Program | Ownership | Target | Indication | 2019 | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|---|---|---|
| BenevolentAI | BEN-8744 | Whole | PDE10 | Ulcerative Colitis | Discovery | Preclinical | Phase 1 | ||
| BEN-28010 | Whole | CHK1 | Glioblastoma Multiforme | Discovery | Preclinical | Preclinical | |||
| BEN-34712 | Whole | RAR⍺β | ALS | Discovery | Preclinical | ||||
| - | Whole | - | Parkinson's disease | Discovery | Discovery | ||||
| - | Whole | - | Fibrosis | Discovery | Discovery | ||||
| Partnered Program | Co-owner w/ AstraZeneca | - | Chronic Kidney Disease | Discovery | |||||
| - | Co-owner w/ AstraZeneca | - | Idiopathic Pulmonary Fibrosis | Discovery | |||||
| - | Co-owner w/ Merck | - | Oncology | Discovery | |||||
| - | Co-owner w/ Merck | - | Neurology | Discovery | |||||
| - | Co-owner w/ Merck | - | Immunology | Discovery | |||||
| BEN-2293 | Whole | TrkA, TrkB, and TrkC | Atopic Dermatitis | Discovery | Preclinical | Phase 1 | Phase 2 | terminated | |
| BEN-9160 | Whole | Bcr-Abl | ALS | Preclinical | |||||
| - | Whole | - | Inflammatory bowl disease (IBD) | Discovery | |||||
| - | Whole | - | Antiviral | Discovery | |||||
| - | Whole | - | Oncology | Discovery | |||||
| - | Whole | - | Oncology | Discovery | |||||
| - | Whole | - | NASH | Discovery | |||||
| - | Whole | - | Oncology | Discovery | |||||
| - | Whole | - | Parkinson's disease | Discovery | |||||
| - | Whole | - | Inflammation | Discovery | |||||
| Exscientia | EXS21546 | Majority, w/ Evotec | A2aR | High Adenosine Signature Cancers | Preclinical | Phase 1 | Phase 1/2 | Phase 1/2 (terminated) | |
| - | Whole | HPK1 | Immuno-Oncology | Discovery | unknown | ||||
| EXS74539 | Whole | LSD1 | Oncology, AML, SCLC | Discovery | Discovery | Preclinical | |||
| EXS73565 | Whole | MALT1 | Oncology, Hematology | Discovery | Discovery | Preclinical | |||
| - | Whole | - | Oncology | Discovery | unknown | ||||
| - | Whole | - | Oncology | Discovery | unknown | ||||
| - | Whole | - | Oncology | Discovery | unknown | ||||
| - | Whole | - | Oncology | Discovery | unknown | ||||
| - | Whole | Mpro | COVID-19 | Discovery | Preclinical | unknown | |||
| - | Whole | - | Anti-infective | Discovery | unknown | ||||
| - | Whole | NLRP3 | Inflammation and Immunity | Discovery | Preclinical | unknown | |||
| GTAEXS617 | Co-owner w/ Apeiron | CDK7 | Transcriptionally addicted cancers | Preclinical | Preclinical | Phase 1/2 | |||
| EXS4318 | Out-licensed, BMS | PKC-theta | inflammatory and immunologic diseases | Preclinical | Preclinical | Preclinical | Phase 1 | ||
| - | Co-owner | - | Oncology | Discovery | Discovery | unknown | |||
| - | Co-owner | ENPP1 | Oncology | Preclinical | Discovery | unknown | |||
| - | Co-owner | ENPP1 | HPP | Preclinical | Discovery | unknown | |||
| - | Co-owner | - | Inframmation and immunity | Discovery | Discovery | unknown | |||
| - | Co-owner | - | Inflammation and Immunity | Discovery | Discovery | unknown | |||
| - | Co-owner | - | Oncology | Discovery | Discovery | unknown | |||
| - | Co-owner | - | Oncology | Discovery | Discovery | unknown | |||
| - | Co-owner | - | Psychiatry | Preclinical | Discovery | unknown | |||
| Insilico Medicine | INS018_055 | Whole | TNIK | IPF | Discovery | Preclinical | Phase 1 | Phase 2 | |
| - | Whole | TNIK | Kidney fibrosis | Discovery | Preclinical | Preclinical | |||
| - | - | TNIK | Skin Fibrosis | Discovery | Discovery | unknown | |||
| - | Whole | TNIK | IPF (inhalable) | Discovery | Discovery | Preclinical | |||
| ISM012 | Whole | PHD1/2 | Anemia of Chronic Kedney Disease | Discovery | Preclinical | Phase 1 | |||
| - | Whole | PHD1/2 | Infammatory bowl disease (IBD) | Discovery | Preclinical | Phase 1 | |||
| ISM8207 | Co-owner w/ Fosun | QPCTL | Immuno-oncology | Discovery | Preclinical | Phase 1 | |||
| - | Co-owner w/ Fosun | - | Diabetic neuphropathy, FSGS | Discovery | unknown | ||||
| ISM3312 | - | 3CLpro | COVID-19 | Discovery | Preclinical | Phase 1 | |||
| ISM3412 | Whole | MAT2A | MTAP-/-cancer | Discovery | Preclinical | Preclinical | |||
| ISM9274 | Whole | CDK12/13 | Solid tumors | Discovery | Discovery | Preclinical | |||
| ISM027 | Whole | cMYC | Solid tumors | Discovery | |||||
| ISM022 | Whole | CDK8 | AML, Solid tumors | Discovery | Discovery | unknown | |||
| ISM023 | Whole | PARP7 | Solid tumors | Discovery | Discovery | unknown | |||
| ISM3091 | Out-licensed, Exelixis | USP1 | BRCA-mutant cancer | Discovery | Preclinical | Phase 1 | |||
| ISM5939 | Whole | ENPP1 | solid tumors | Discovery | Discovery | Preclinical | |||
| ISM5043 | Out-licensed, Menarini | KAT6 | ER+/HER2-breast cancer | Preclinical | |||||
| ISM4525 | Whole | DGKA | Solid tumors | Preclinical | |||||
| ISM8001 | Whole | FGFR2/3 | Solid tumors | Preclinical | |||||
| ISM016 | Whole | NLRP3 | Gout flare | Discovery | Discovery | Discovery | |||
| ISM6331 | Whole | TEAD | Solid tumors | Preclinical | |||||
| Recursion Pharmaceuticals | REC-4881 | Whole | MEK1 and MEK2 | Familial Adenomatous Polyposis | Preclinical | Phase 1 | Phase 1 | Phase 2 | |
| REC-3599 | Whole | PKC and GSK3ß | GM2 Gangliosidosis | Preclinical | Phase 1 | terminated | |||
| REC-2282 | Whole | HDAC | Neurofibromatosis Type 2 | Preclinical | Phase 1 | Phase 1 | Phase 3 | ||
| REC-994 | Whole | antioxidant, no specific target | Cerebral Cavemous Malformation | Preclinical | Phase 1 | Phase 1 | Phase 2 | ||
| REC-3964 | Whole | - | Clostridium Difficile Colitis | Discovery | Preclinical | Preclinical | Phase 1 | ||
| - | Whole | - | Neuroinflammation | Discovery | Discovery | unknown | |||
| - | Whole | - | Batten Disease | Discovery | unknown | ||||
| - | Whole | - | Charcot-Marie-Tooth Disease Type 2 | Discovery | Discovery | unknown | |||
| - | Whole | - | Immune Checkpoint resistance in STK11-NSCLC | Preclinical | Preclinical | unknown | |||
| - | Whole | - | Oncoloty | Discovery | Discovery | Discovery | |||
| 25 programs | - | - | Various | Preclinical | unknown | ||||
| REC-4881 | Whole | - | AXIN1 or APC Mutant Cancers | Phase 1 | |||||
| - | - | - | Pulmonary Arterial Hypertension | Preclinical | unknown | ||||
| - | Whole | - | - | Preclinical | unknown | ||||
| Immunotherapy Target Alpha | Whole | - | Oncology | Discovery | Discovery | ||||
| Immunotherapy Target Beta | Whole | - | Oncology | Discovery | unknown | ||||
| - | Whole | - | Hepatocellular Carcinoma | Discovery | unknown | ||||
| - | Whole | RBM39 | HR-proficient Ovarian Cancer RBM39 | Preclinical | |||||
| Immunotherapy Target Delta | Whole | - | - | Preclinical | |||||
| Relay Therapeutics | RLY-4008 | Whole | FGFR2 (mutant+WT) | FGFR2-altered cholangiocarcinoma (CCA) | Discovery | Phase 1 | Phase 1 | Phase 1 | Phase 1/2 |
| RLV-PI3K1047 (RLY-5836) | Whole | PI3Kα | - | Discovery | Preclinical | Phase 1 | |||
| RLY2608 | Whole | PI3Kα | solid tumors with a PI3Kα mutation | Phase 1 | Phase 1 | ||||
| - | Whole | PI3Kα | - | Discovery | unknown | ||||
| RLY-2139 | Whole | CDK2 | Oncology | Discovery | Discovery | unknown | |||
| - | Whole | ERα | - | unknown | |||||
| GDC-1971 | Co-owner w/ Genentech | SHP2 | Cancers, expand into multiple combination | Preclinical | Phase 1 | Phase 1 | Phase 1 | Phase 1 | |
| - | Whole | - | Oncology | Discovery | Discovery | unknown | |||
| - | Whole | - | Oncology | Discovery | Discovery | unknown | |||
| - | Whole | - | Genetic disease | Discovery | Discovery | unknown | |||
| - | Whole | - | Genetic disease | Discovery | Discovery | unknown | |||
| Schrödinger | SDGR3 (SGR-1505) | Whole | MALT1 | Relapsed or refractory B-cell lymphoma, chronic lymphocytic leukemia | Discovery | Discovery | Preclinical | Phase 1 | Phase 1 |
| SDGR1 | Whole | CDC7 | Esophagial and Lung Cancers, | Discovery | Discovery | Discovery | unknown | ||
| SGR-2921 | Whole | CDC7 | Hematological cancers and solid tumors | Preclinical | Phase 1 | ||||
| SDGR2 | Whole | WEE1 | Ovarian, Pancreatic, Breast and Lung Cancers | Discovery | Discovery | Discovery | unknown | ||
| SGR-3515 | Whole | WEE1/MYT1 | Solid tumors | Discovery | Preclinical | ||||
| SDGR4 | Co-owner w/ BMS | HIF-2a | Renal Cell Carcinoma | Discovery | Discovery | unknown | |||
| SDGR5 | Co-owner w/ BMS | SOS1 | KRAS-driven Cancers | Discovery | Discovery | Discovery | Preclinical | ||
| - | Co-owner w/ BMS | - | Oncology, Immunology, Neurology | Discovery | unknown | ||||
| - | Co-owner w/ BMS | - | Neurology | Discovery | |||||
| - | Co-owner w/ BMS | - | immunology | ||||||
| - | Whole | LRRK2 | Neurology | Discovery | Discovery | ||||
| - | Whole | PRMT5-MTA | Oncology | Discovery | Discovery | ||||
| - | Whole | EFGR(C797S) | Oncology | Discovery | Discovery | ||||
| - | Whole | - | Oncology | Discovery | Discovery | ||||
| - | Whole | - | Oncology | Discovery | Discovery | ||||
| - | Whole | NLRP3 | Immunology | Discovery | Discovery | ||||
| - | Whole | - | Immunology | Discovery | Discovery | ||||
| - | Co-owner w/ BMS | - | Oncology | Discovery | unknown | ||||
| - | Co-owner w/ BMS | - | Oncology | Discovery | unknown | ||||
| - | Co-owner w/ BMS | - | Immunology | Discovery | unknown | ||||
| - | Co-owner w/ BMS | - | Oncology, Immunology, Neurology | Discovery | Discovery | ||||
| - | Co-owner w/ Takeda | - | Oncology | Discovery | Discovery | ||||
| - | Co-owner w/ Takeda | TYK2 | Psoriasis | Phase 2 | |||||
| - | Co-owner w/ Zai Lab | - | Oncology | Discovery | unknown | ||||
| - | Co-owner w/ Lilly | - | Immunology | Discovery | Discovery | ||||
| - | Co-owner w/ Ajax | JAK2 | Oncology | Discovery | |||||
| - | Co-owner w/ Bright Angel Therapeutics | HSP90 | Antifungal | Discovery | |||||
| - | Co-owner w/ loxo Therapeutics | undisclosed | oncology | Phase 1 | |||||
| - | Co-owner w/ Morphic Therapeutic | α4β7 | IBD | Phase 2 | |||||
| - | Co-owner w/ Morphic Therapeutic | α4β7 | GI indications | Discovery | |||||
| - | Co-owner w/ Morphic Therapeutic | αvβ8 | Solid tumors, fibrosis | Discovery | |||||
| - | Co-owner w/ Morphic Therapeutic | - | Pulmonary arterial hypertension | Discovery | |||||
| - | Co-owner w/ NimbusTherapeutic | HPK1 | Immuno-oncology | Phase 1/2 | |||||
| - | Co-owner w/ Otsuka | - | - | Discovery | |||||
| - | Co-owner w/ Sanofi | - | oncology | Discovery | |||||
| - | Co-owner w/ Structure Therapeutics | APJR | Pulmonary arterial hypertension | Phase 1 | |||||
| - | Co-owner w/ Structure Therapeutics | - | - | Discovery | |||||
| - | Co-owner w/ Structure Therapeutics | LPA1R | Idiopathic pulmonary fibrosis | Preclinical | |||||
| Insitro | - | Co-owner w/ BMS, TSC alliance | - | Genetic Epilepsies, ALS | Discovery | ||||
| - | Whole | - | Solid Tumors | Discovery | |||||
| - | Whole | - | MASLD, Obesity | Discovery | |||||
| Verge Genomics | VRG50635 | Whole | PIKfyve | ALS | Discovery | Preclinical | Phase 1 | Phase 1/2 | |
| - | Whole | - | Parkinson's Disease | Discovery | Discovery | ||||
| - | Whole | - | Parkinson's Disease | Discovery | unknown | ||||
| - | Whole | - | Parkinson's Disease | Discovery | unknown | ||||
| - | Whole | - | Frontotemporal Dementia | Discovery | unknown | ||||
| - | Whole | - | Progressive Supranuclear Palsy | Discovery | unknown | ||||
| - | Whole | - | Schizophrenia | Discovery | Discovery | ||||
| - | Whole | - | Neurodegenerative Diseases | Discovery | Discovery | ||||
| - | Whole | - | Undisclosed | Discovery | unknown | ||||
| - | Whole | PIKfyve | COVID-19 | Discovery | Preclinical | unknown | |||
| - | - | - | Psoriasis | Discovery | |||||
| - | - | - | Atopic Dermititis | Discovery | |||||
| - | - | - | Crohn's Disease | Discovery | |||||
| - | - | - | Ulcerative Colitis | Discovery | |||||
| Partnered Programs | Co-owner w/ Lilly | - | ALS | Discovery | |||||
| - | Co-owner w/ Alexion | - | Neurodegenerative Diseases | Discovery | |||||
| - | Co-owner w/ Alexion | - | Neuromuscular Diseases | Discovery | |||||
| Valo Health | OPL-0301 | - | S1P1 agonist | Heart failure and Acute Kidney Injury | Phase 1 | Phase 2 | Phase 2 | ||
| OPL-0401 | - | ROCK 1/2 inhibitor | Diabetic Retinopathy | Phase 1 | Phase 2 | Phase 2 | |||
| OPAL-0022 | - | - | Atherosclerosis | Discovery | unknown | ||||
| OPAL-0004 | - | - | Atherosclerosis, Giloblastoma | Discovery | unknown | ||||
| OPAL-0018 | - | - | Atherosclerosis | Discovery | unknown | ||||
| OPAL-0003 | - | - | Heart Failure, Giloblastoma | Discovery | unknown | ||||
| OPL-0101 | - | - | Immuno-Oncology | Discovery | Preclinical | unknown | |||
| OPAL-0021 | - | - | cancer | Discovery | unknown | ||||
| OPAL-0015 | - | USP28 | NSCLC, Squamous Cell Carcinoma, Targeted Defined Tumors | Discovery | unknown | ||||
| OPAL-0024 | - | - | Solid Tumors | Discovery | unknown | ||||
| OPAL-0001 | - | PARP1 | Medula/Glioblastoma Brain Tumors, Breast Cancer | Discovery | unknown | ||||
| OPAL-0014 | - | - | Pancreatic Ductal Adenocarcinoma (PDAC), Targeted Defined Tumors | Discovery | unknown | ||||
| OPAL-0023 | - | - | Defined Tumors, Immune Modulation | Discovery | unknown | ||||
| OPAL-0012 | - | USP7 | NSCLC | Discovery | unknown | ||||
| OPAL-0016 | - | - | Induced Neuropathy and Cardiomyopathy | Discovery | unknown | ||||
| OPAL-0002 | - | - | Neurodegenerative disorders | Discovery | unknown | ||||
| OPAL-0006 | - | - | Neurodegenerative: Oncology (metastatic) | Discovery | unknown |
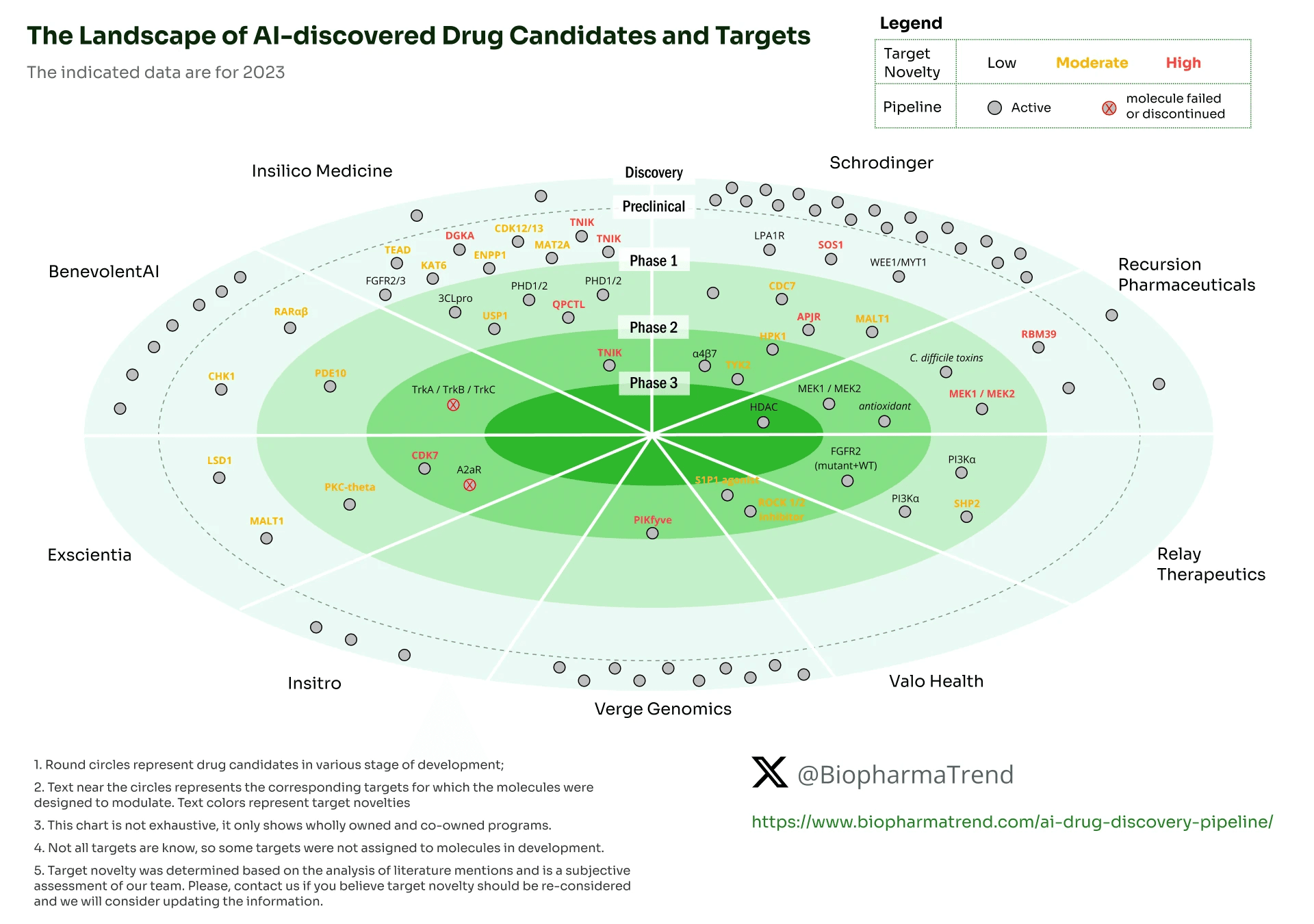
요약
- 전체적인 임상 진입 파이프라인의 수는 schrodinger 가 압도적으로 많습니다.
- AI 기반의 물질 발굴에서 가장 기대할 수 있는 부분인 target novelty를 반영할 경우 Insilico Medicine의 TNIK 저해제 개발을 선두로 볼 수 있습니다.
- Recursion의 경우에도 임상 2상/3상이 동시 진행되고 있어 선두 그룹으로 볼 수 있지만, 타깃의 신규성 측면에선 Insilico Medicine의 연구 결과가 가장 기대됩니다.
- 초기 AI 기업인 Exscientia & Benevolent는 각각 한 번의 실패를 경험했기에 소극적인 활동을 보입니다.
- 사실상 아직 AI로 발굴된 물질 중에서는 임상 2상을 통과한 경우가 없다고 볼 수 있습니다.
- AI는 discovery 단계에서 물질 발굴의 속도를 비약적으로 높여주는 것은 검증되었지만, 아직 임상에서의 성공으로 이어지지는 않는 모습입니다.
결론
AI가 후보 물질 discovery에서의 개발 기간 단축 및 개발 비용 절감에 큰 역할을 하면서 여러 AI 기반 신규 물질이 등장하고 있지만, 임상에서의 PoC(Proof-of-Concept)를 입증한 물질은 아직 없습니다. 여전히 시장이 성숙하지 못했고, 선두 그룹의 Follow-up이 필요한 단계입니다. 이는 AI 기술이 만능이 아니며, AI 기술을 뒷받침할 또 다른 역량이 필요하다는 점을 시사합니다.
즉, AI 소프트웨어와 바이오의 유기적인 결합은 AI 기업의 독립적인 행보만으로는 부족하며, 여러 바이오 기업과의 협력을 통해서야 비로소 가능하다고 보입니다. 성공적인 신약 개발의 선두 그룹에 히츠의 하이퍼랩이 중요한 도구로 사용되길 바랍니다.